Dynamics in crystalline and non-crystalline ice
Water is one of the seemingly most ordinary substances. Nevertheless, many of its unusual properties, the so-called anomalies, are not yet understood in detail. We are investigating the polymorphism of water: In solid form - i.e., as ice - water molecules can combine to form a wide variety of crystalline and amorphous structures; about 20 of these have now been elucidated. This distinguishes ice from most other materials, where only one or a few molecular arrangements are known. In recent years, numerous new ice phases have been discovered, but the phase diagram of ice is far from complete. This is because although computer simulations predict additional structures, they are often difficult to realize experimentally because the time scales on which phase transitions occur can be long. Also, many structures are metastable and thus can only be produced via complex multistep preparation protocols that must first be discovered.
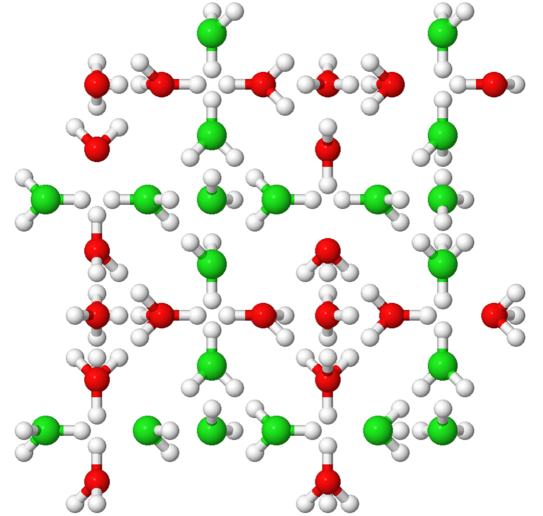
We study reorientation processes of water molecules in different ice phases using electrical impedance spectroscopy as well as nuclear magnetic resonance. These methods allow us to observe phase transitions and determine the time scales on which molecular motions occur.
Articles on "Dynamics in crystalline and non-crystalline ice".